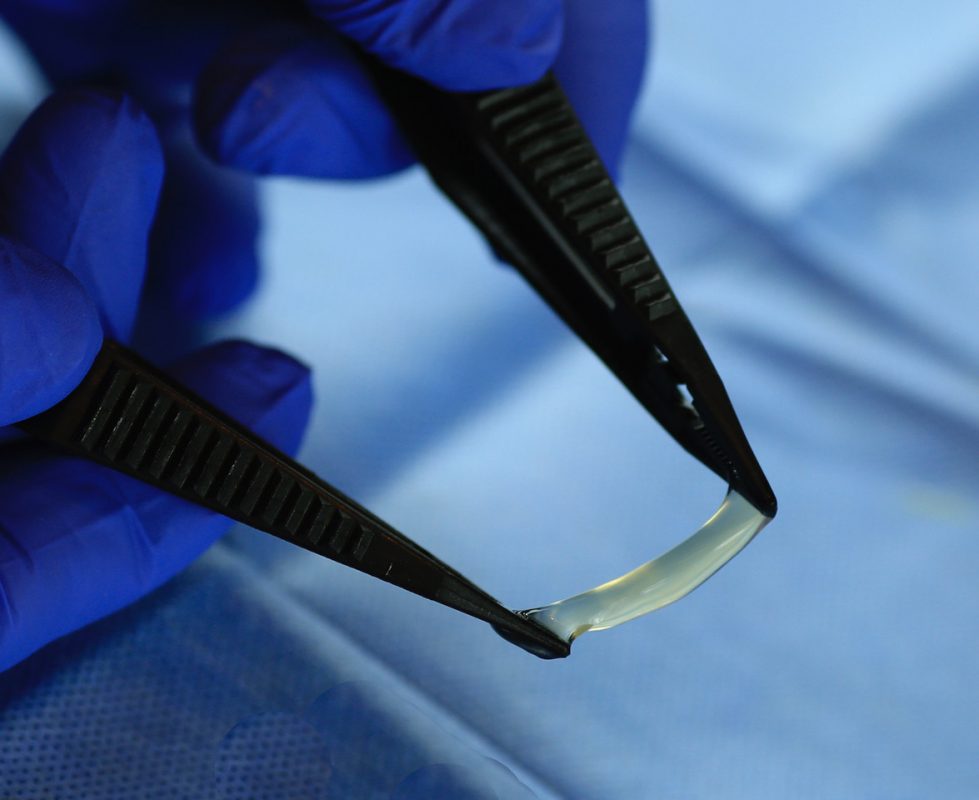
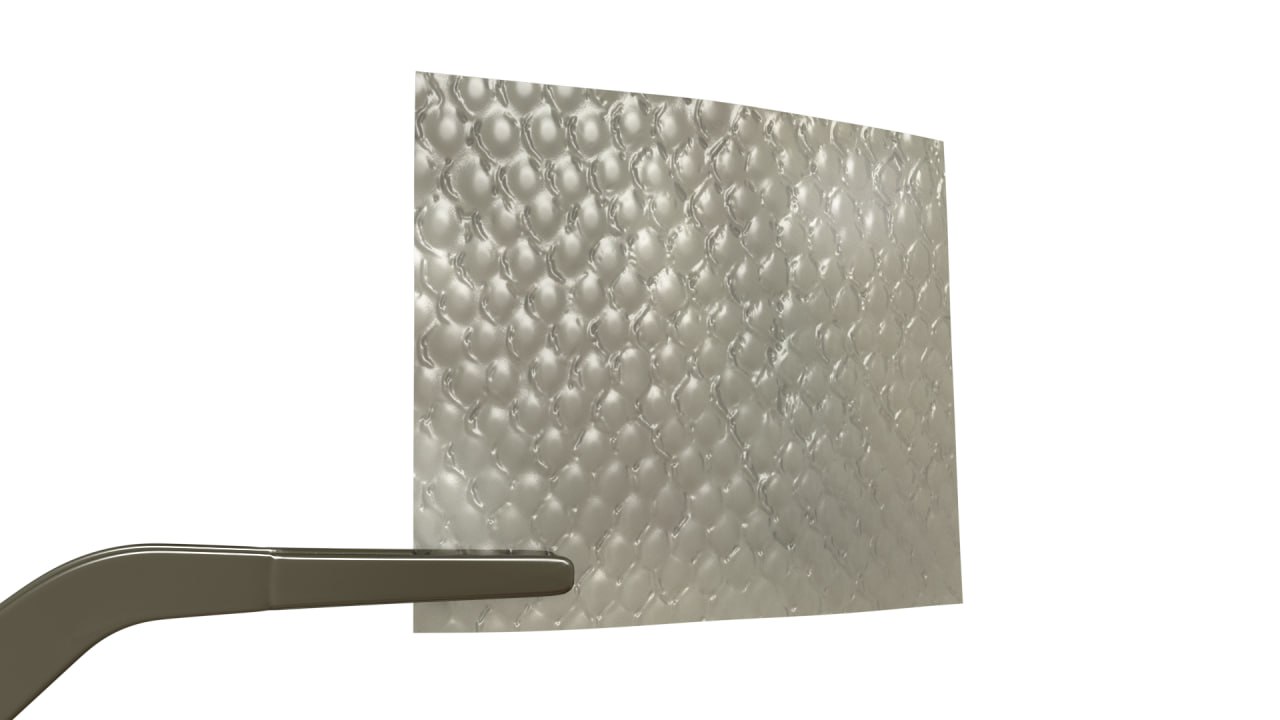
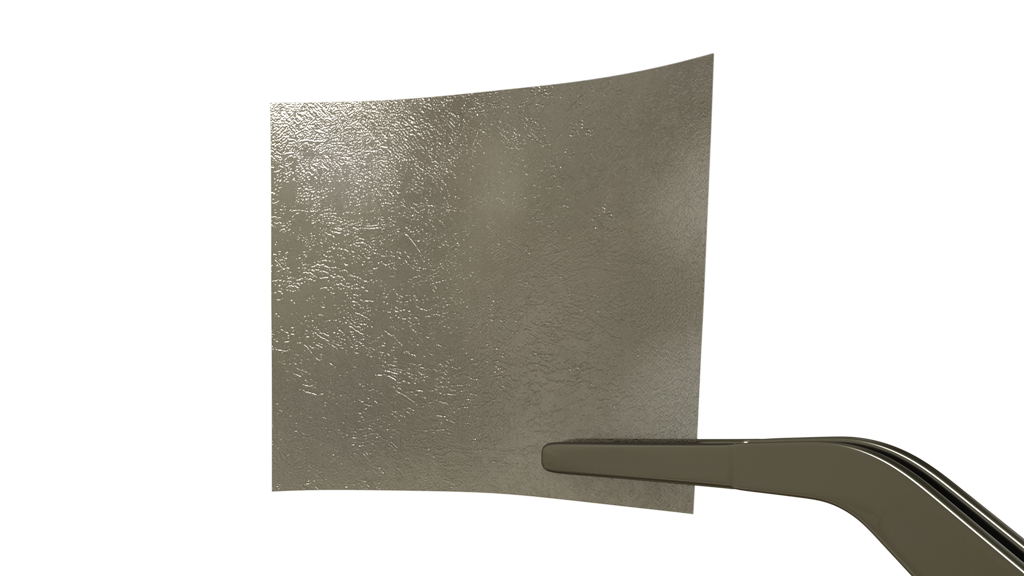
completeFT™ is our next generation placental membrane for homologous use. A multi-layer graft processed with an advanced proprietary processing technology to ensure that all naturally occurring components of each layer of the placental tissue remains in-tact.
completeFT™ is a Full Thickness graft, containing the Amnion, the Intermediate / Spongy layer as well as the Chorion layer of the placenta. This may provide additional growth factors, extracellular matrix, and proteins. The tissue is minimally manipulated only and is delivered at ambient temperature with a 5-year shelf life.
completeFT™ is homologous use because serving as a natural covering and offering protection from the surrounding environment are the inherent functions of amniotic membrane.